由 sufang 在 日, 08/03/2014 – 09:28 發表 Pre-published Cancer epigenomics Folate MATHFR Methionine Methylation SAM
重要文獻 (Research Notes: Cacner epigenomics (folate))
1. Kane, M. A. (2005) The role of folates in squamous cell carcinoma of the head and neck. Cancer detection and prevention 29, 46-53. (pdf 4099.) The primary objective of this review is to explore the hypothesis that folate insufficiency may be important in the pathogenesis of squamous cell carcinomas of the head and neck (SCCHN) and that folate repletion may be an effective component of chemoprevention. The main results are that folate insufficiency disrupts DNA global and specific gene methylation patterns such that the activity of certain tumor suppressor genes such as p16 and possibly p53 may be lost. Folate pool imbalance and impaired repair mechanisms may contribute to DNA instability and strand breaks. Sensitive methods exist for identification of individuals with folate insufficiency in contrast to the relatively insensitive conventional serum or red cell folate assays with broad “normal” ranges. The impact of folate supplementation can thus be quantified. Folate imbalance may result from alterations in folate cellular uptake by the reduced folate carrier (RFC) and/or the folate receptor (FR) and polymorphisms in enzymes important in folate retention such as folylpolyglutamate synthetase and in folate modification such as methylene tetrahydrofolate reductase (MTHFR). Known predisposing factors for SCCHN such as alcohol and tobacco carcinogens may influence folate balance. Folate supplementation may reduce primary or secondary risk of cancer. Formal studies of folate sufficiency in persons at risk for or diagnosed and treated for SCCHN are needed to define the role of folate supplementation in the prevention of these cancers.
2. Kraunz, K. S., D. Hsiung, M. D. McClean, M. Liu, J. Osanyingbemi, H. H. Nelson, and K. T. Kelsey. (2006) Dietary folate is associated with p16(INK4A) methylation in head and neck squamous cell carcinoma. Int J Cancer 119, 1553-7. (pdf 4098.) Inactivation of the p16(INK4A) (CDKN2A) gene in the Rb pathway is among the most common somatic alterations observed in tobacco-related solid tumors, including head and neck squamous cell carcinoma (HNSCC). In addition, a low folate diet is an important risk factor for HNSCC. Decreased dietary folate in an animal model of hepatocellular carcinoma has been associated with the induction of epigenetic silencing of the p16(INK4A) gene. In an ongoing population-based study of HNSCC, we sought to extend this observation to human disease testing the hypothesis that p16(INK4A) methylation is associated with decreased dietary folate. We also investigated the association of methylation silencing with functional polymorphisms in the folate metabolism enzyme methylene tetrahydrofolate reductase (MTHFR). In 169 HNSCCs, the odds ratio for p16(INK4A) methylation among those with low dietary folate intake was 2.3 (95% CI = 1.1-4.8) when compared with those with high folate intake. Furthermore, this increased risk for epigenetic silencing at p16(INK4A) was modified by the MTHFR alleles previously associated with diminished serum folate levels. Hence, in HNSCC low dietary intake of folate is associated with p16(INK4A) methylation, and this relationship is modified by the MTHFR genotype. Our data provides important evidence for a mechanism of action of folate deficiency in cancer.
3. Ulrich, C. M. (2007) Folate and cancer prevention: a closer look at a complex picture. The American journal of clinical nutrition 86, 271-3. 一刀二刃。 (pdf 4100)
4. Berdasco, M., and M. Esteller. (2010) Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell 19, 698-711. (pdf 2904 Must Read. (was referred by Hanahan and Weinberg)) Appropriate patterns of DNA methylation and histone modifications are required to assure cell identity, and their deregulation can contribute to human diseases, such as cancer. Our aim here is to provide an overview of how epigenetic factors, including genomic DNA methylation, histone modifications, and microRNA regulation, contribute to normal development, paying special attention to their role in regulating tissue-specific genes. In addition, we summarize how these epigenetic patterns go awry during human cancer development. The possibility of “resetting” the abnormal cancer epigenome by applying pharmacological or genetic strategies is also discussed.
5. Feil, R., and M. F. Fraga. (2011) Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet 13, 97-109. (pdf 3200.) Epigenetic phenomena in animals and plants are mediated by DNA methylation and stable chromatin modifications. There has been considerable interest in whether environmental factors modulate the establishment and maintenance of epigenetic modifications, and could thereby influence gene expression and phenotype. Chemical pollutants, dietary components, temperature changes and other external stresses can indeed have long-lasting effects on development, metabolism and health, sometimes even in subsequent generations. Although the underlying mechanisms remain largely unknown, particularly in humans, mechanistic insights are emerging from experimental model systems. These have implications for structuring future research and understanding disease and development.
6. Crider, K. S., T. P. Yang, R. J. Berry, and L. B. Bailey. (2012) Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr 3, 21-38. (pdf 4146 in BB_Cancer Epigenomics.) DNA methylation is an epigenetic modification critical to normal genome regulation and development. The vitamin folate is a key source of the one carbon group used to methylate DNA. Because normal mammalian development is dependent on DNA methylation, there is enormous interest in assessing the potential for changes in folate intake to modulate DNA methylation both as a biomarker for folate status and as a mechanistic link to developmental disorders and chronic diseases including cancer. This review highlights the role of DNA methylation in normal genome function, how it can be altered, and the evidence of the role of folate/folic acid in these processes.
7. Gut, P., and E. Verdin. (2013) The nexus of chromatin regulation and intermediary metabolism. Nature 502, 489-98. (pdf 3881.) Living organisms and individual cells continuously adapt to changes in their environment. Those changes are particularly sensitive to fluctuations in the availability of energy substrates. The cellular transcriptional machinery and its chromatin-associated proteins integrate environmental inputs to mediate homeostatic responses through gene regulation. Numerous connections between products of intermediary metabolism and chromatin proteins have recently been identified. Chromatin modifications that occur in response to metabolic signals are dynamic or stable and might even be inherited transgenerationally. These emerging concepts have biological relevance to tissue homeostasis, disease and ageing.
8. Zenobi, R. (2013) Single-cell metabolomics: analytical and biological perspectives. Science 342, 1243259. (Zenobi, R 2013 Dec 6;342(6163):1243259. ) There is currently much interest in broad molecular profiling of single cells; a cell’s metabolome-its full complement of small-molecule metabolites-is a direct indicator of phenotypic diversity of single cells and a nearly immediate readout of how cells react to environmental influences. However, the metabolome is very difficult to measure at the single-cell level because of rapid metabolic dynamics, the structural diversity of the molecules, and the inability to amplify or tag small-molecule metabolites. Measurement techniques including mass spectrometry, capillary electrophoresis, and, to a lesser extent, optical spectroscopy and fluorescence detection have led to impressive advances in single-cell metabolomics. Even though none of these methodologies can currently measure the metabolome of a single cell completely, rapidly, and nondestructively, progress has been sufficient such that the field is witnessing a shift from feasibility studies to investigations that yield new biological insight. Particularly interesting fields of application are cancer biology, stem cell research, and monitoring of xenobiotics and drugs in tissue sections at the single-cell level.
In recent years, there has been a surge in the development and application of single-cell molecular profiling [for reviews with a focus on metabolomics, see (2–8)].
In recent years, there has been a surge in the development and application of single-cell molecular profiling [for reviews with a focus on metabolomics, see (2–8)].
9. Fanidi, A., C. Relton, P. M. Ueland, O. Midttun, S. E. Vollset, R. C. Travis, A. Trichopoulou, P. Lagiou, D. Trichopoulos, H. B. Bueno-de-Mesquita, M. Ros, H. Boeing, R. Tumino, S. Panico, D. Palli, S. Sieri, P. Vineis, M. J. Sanchez, J. M. Huerta, A. Barricarte Gurrea, L. Lujan-Barroso, J. R. Quiros, A. Tjonneland, J. Halkjaer, M. C. Boutron-Ruault, F. Clavel-Chapelon, C. Cadeau, E. Weiderpass, M. Johansson, E. Riboli, P. Brennan, and M. Johansson. (2014) A prospective study of one-carbon metabolism biomarkers and cancer of the head and neck and esophagus. Int J Cancer (Pdf 4093.) Experimental and epidemiological data suggest that factors of one-carbon metabolism are important in the pathogenesis of several cancers, but prospective data on head and neck cancer (HNC) and esophagus cancer are limited. The European Prospective Investigation into Cancer and Nutrition (EPIC) study recruited 385,747 participants from 10 countries who donated a blood sample. The current study included 516 cancer cases of the head and neck and esophagus and 516 individually matched controls. Plasma levels of vitamins B2, B6, B9 (folate), B12, and methionine and homocysteine were measured in pre-diagnostic plasma samples and analyzed in relation to HNC and esophagus cancer risk, as well as post-diagnosis all-cause mortality. After controlling for risk factors, study participants with higher levels of homocysteine had elevated risk of HNC, the odds ratio (OR) in conditional analysis when comparing the top and bottom quartiles of homocysteine [ORQ4vs.Q1] being 2.13 (95% confidence interval [95% CI] 1.13-4.00, P for trend 0.009). A slight decrease in HNC risk was also seen among subjects with higher levels of folate (ORQ4vs.Q1 0.63, 95% CI 0.35-1.16, P for trend 0.02). Subgroup analyses by anatomical sub-site indicated particularly strong associations with circulating homocysteine for oral cavity and gum cancer (P for trend 8×10-4 ), as well as for oropharynx cancer (P for trend 0.008). Plasma concentrations of the other investigated biomarkers did not display any clear association with risk or survival. In conclusion, study participants with elevated circulating levels of homocysteine had increased risk of developing squamous cell carcinoma of the head and neck. (c) 2014 Wiley Periodicals, Inc.
10. Galeone, C., V. Edefonti, M. Parpinel, E. Leoncini, K. Matsuo, R. Talamini, A. F. Olshan, J. P. Zevallos, D. M. Winn, V. Jayaprakash, K. Moysich, Z. F. Zhang, H. Morgenstern, F. Levi, C. Bosetti, K. Kelsey, M. McClean, S. Schantz, G. P. Yu, P. Boffetta, Y. C. Amy Lee, M. Hashibe, C. La Vecchia, and S. Boccia. (2014) Folate intake and the risk of oral cavity and pharyngeal cancer: A pooled analysis within the INHANCE Consortium. Int J Cancer (Pdf 4092.) There are suggestions of an inverse association between folate intake and serum folate levels and the risk of oral cavity and pharyngeal cancers (OPC), but most studies are limited in sample size, with only few reporting information on the source of dietary folate. This study aims to investigate the association between folate intake and the risk of OPC within the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. We analyzed pooled individual-level data from 10 case-control studies participating in the INHANCE consortium, including 5,127 cases and 13,249 controls. Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were estimated for the associations between total folate intake (natural, fortification and supplementation) and natural folate only, and OPC risk. We found an inverse association between total folate intake and overall OPC risk (the adjusted OR for the highest versus the lowest quintile was 0.65, 95% CI: 0.43-0.99), with a stronger association for oral cavity (OR=0.57, 95% CI: 0.43-0.75). A similar inverse association, though somewhat weaker, was observed for folate intake from natural sources only (OR=0.64, 95% CI: 0.45-0.91). The highest OPC risk was observed in heavy alcohol drinkers with low folate intake as compared to never/light drinkers with high folate (OR=4.05, 95% CI: 3.43-4.79); the attributable proportion due to interaction was 11.1%(95% CI: 1.4-20.8%). The present project of a large pool of case-control studies supports a protective effect total folate intake on OPC risk. (c) 2014 Wiley Periodicals, Inc.
#1由 sufang 在 一, 08/04/2014 – 09:37 發表。
Untitled Folder_20140708
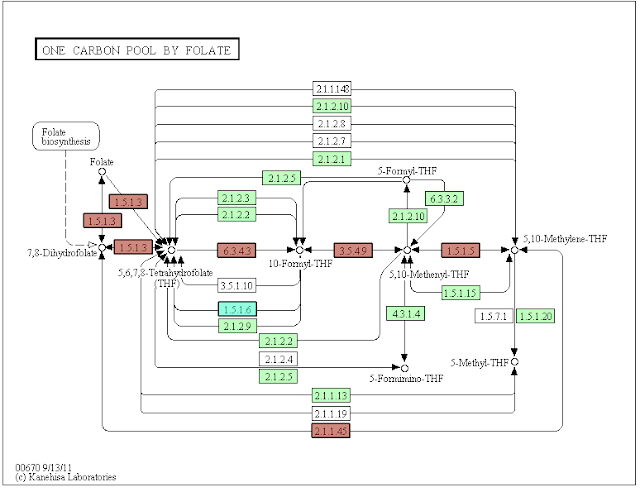
One Carbon Pool_NNK short
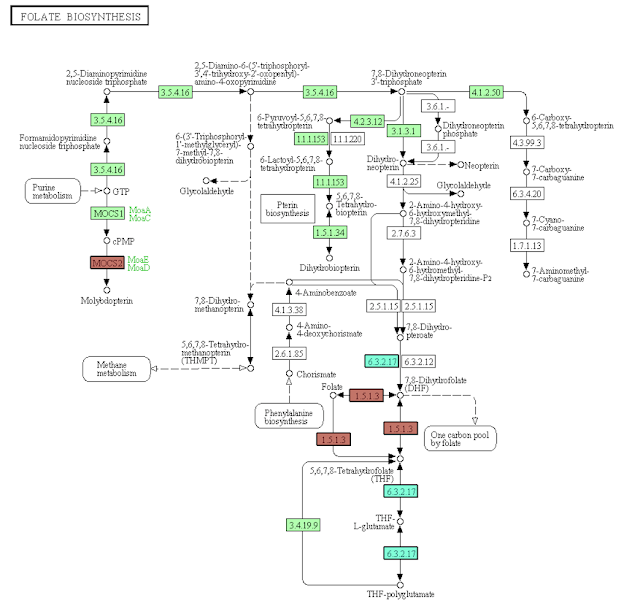
Folate_Biosynthesis_ACshort